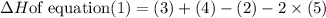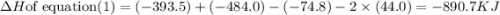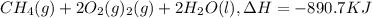Click on the hess's law of constant heat summation button within the activity and use the example shown to calculate the reaction enthalpy, δh, for the following reaction: ch4(g)+2o2(g)→co2(g)+2h2o(l)use the series of reactions that follow: c(s)+2h2(g)→ch4(g), δh =−74.8 kj. c(s)+o2(g)→co2(g), δh =−393.5 kj. 2h2(g)+o2(g)→2h2o(g), δh =−484.0 kj. h2o(l)→h2o(g), δh =44.0 kj.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Click on the hess's law of constant heat summation button within the activity and use the example sh...
Questions


Spanish, 13.10.2020 08:01


Advanced Placement (AP), 13.10.2020 08:01

Computers and Technology, 13.10.2020 08:01



Mathematics, 13.10.2020 08:01


History, 13.10.2020 08:01

English, 13.10.2020 08:01

Health, 13.10.2020 08:01

Mathematics, 13.10.2020 08:01



Business, 13.10.2020 08:01

Mathematics, 13.10.2020 08:01

History, 13.10.2020 08:01

English, 13.10.2020 08:01

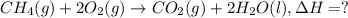 ...(1)
...(1)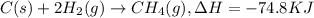 ...(2)
...(2)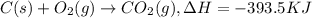 ...(3)
...(3)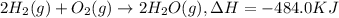 ...(4)
...(4)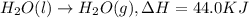 ...(5)
...(5)