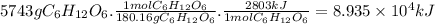Chemistry, 27.07.2019 13:00 rakanmadi87
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +2803 kj/mol calculate the solar energy required to produce 5743 g of c6h12o6.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +...
Questions

Spanish, 11.12.2020 06:30

Mathematics, 11.12.2020 06:30



English, 11.12.2020 06:30


Mathematics, 11.12.2020 06:30

Mathematics, 11.12.2020 06:30

Mathematics, 11.12.2020 06:30



Biology, 11.12.2020 06:30

History, 11.12.2020 06:30


Mathematics, 11.12.2020 06:30

Mathematics, 11.12.2020 06:30