
Chemistry, 27.07.2019 13:00 Clervoyantyvonne
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) according to one acid-base theory, the forward reaction is classified as an acid-base reaction because

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) ac...
Questions











Mathematics, 29.05.2020 20:07



Mathematics, 29.05.2020 20:07






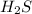 loses proton and
loses proton and 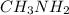 accepts proton
accepts proton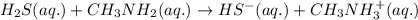
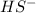 which is a conjugate base.
which is a conjugate base.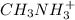 which is a conjugate acid.
which is a conjugate acid.

