Chemistry, 27.07.2019 12:30 rissaroo159
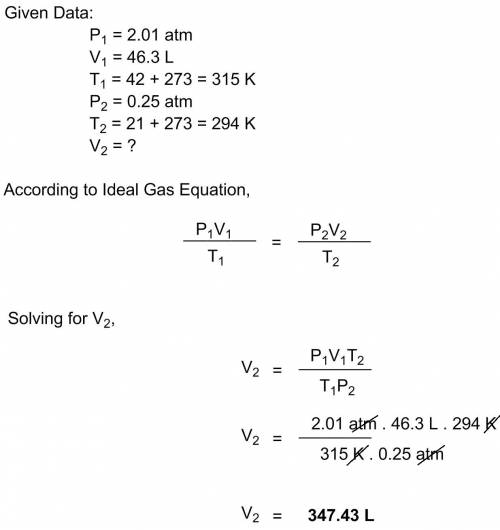
Aballoon contains 46.3 l of helium at 42 oc, and 2.01 atm is released into the air. at a certain altitude, the temperature falls to 21 oc, and the pressure falls to 0.25 atm. what is the volume of the balloon under these conditions? a. 3.5 l b. 35.0 l c. 347.5 l d. 3475.0 l e. none of the above

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Aballoon contains 46.3 l of helium at 42 oc, and 2.01 atm is released into the air. at a certain alt...
Questions

Computers and Technology, 03.05.2021 23:20

Mathematics, 03.05.2021 23:20



English, 03.05.2021 23:20

Mathematics, 03.05.2021 23:20

Biology, 03.05.2021 23:20


Mathematics, 03.05.2021 23:20




Chemistry, 03.05.2021 23:20

English, 03.05.2021 23:20

Mathematics, 03.05.2021 23:20



Social Studies, 03.05.2021 23:20


Health, 03.05.2021 23:20