

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
The nickel(ii) ion is commonly dissolved in solution using nickel(ii) nitrate hexahydrate, ni(no3)2....
Questions

Mathematics, 13.01.2021 22:50


Mathematics, 13.01.2021 22:50

Mathematics, 13.01.2021 22:50






Mathematics, 13.01.2021 22:50

Spanish, 13.01.2021 22:50


Mathematics, 13.01.2021 22:50


English, 13.01.2021 22:50



Chemistry, 13.01.2021 22:50

Physics, 13.01.2021 22:50

Mathematics, 13.01.2021 22:50

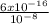 = 6 x 10⁻⁸ M
= 6 x 10⁻⁸ M

