
Chemistry, 27.07.2019 12:00 thegent1859
Asaturated solution of ag2cro4 has a cro42- concentration of 6.7 x 10-5 m. calculate the molarity of ag+ if the ksp for ag2cro4 is equal to 1.2 x 10-12

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Asaturated solution of ag2cro4 has a cro42- concentration of 6.7 x 10-5 m. calculate the molarity of...
Questions


Mathematics, 03.02.2020 08:53


Health, 03.02.2020 08:53

Mathematics, 03.02.2020 08:53


Mathematics, 03.02.2020 08:53

History, 03.02.2020 08:53

Social Studies, 03.02.2020 08:53


Biology, 03.02.2020 08:53

Physics, 03.02.2020 08:53


History, 03.02.2020 08:53

Mathematics, 03.02.2020 08:53




Mathematics, 03.02.2020 08:53

English, 03.02.2020 08:53

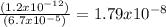
![[Ag^{+}] = \sqrt{1.79 x 10^{-8}} = 1.35 x 10^{-4}](/tpl/images/0138/7009/3c747.png)


