Chemistry, 27.07.2019 08:30 xxxamslashxxx9
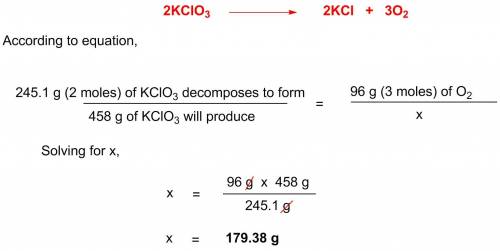
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2, if you start with 458 g of kclo3, what mass (g) of o2 will be produced? a. 83.71 l o2 b. 179.39 l o2 c. 687 l o2 d. 67.2 l o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2...
Questions


English, 09.11.2020 22:50

Mathematics, 09.11.2020 22:50



Mathematics, 09.11.2020 22:50



English, 09.11.2020 22:50

Physics, 09.11.2020 22:50

SAT, 09.11.2020 22:50


Chemistry, 09.11.2020 22:50

History, 09.11.2020 22:50

English, 09.11.2020 22:50


Mathematics, 09.11.2020 22:50



Mathematics, 09.11.2020 22:50