Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1
You know the right answer?
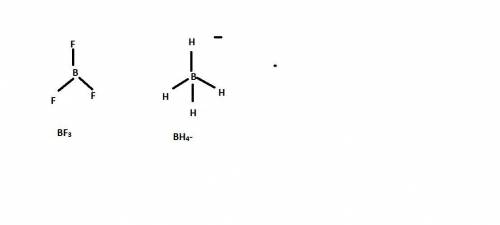
Sodium borohydride, nabh4, and boron trifluoride, bf3, are compounds of boron. what are the shapes a...
Questions








History, 04.04.2020 05:59



Mathematics, 04.04.2020 05:59







Mathematics, 04.04.2020 06:00

Mathematics, 04.04.2020 06:00

Social Studies, 04.04.2020 06:00

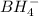 is tetrahedral and in boron trifluoride
is tetrahedral and in boron trifluoride 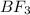 is trigonal planar.
is trigonal planar.![:{\text{Number of electron pairs}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0137/9746/ebbe5.png)
![{\text{Number of electrons}} =\frac{1}{2}[3+4-0+1]=4](/tpl/images/0137/9746/1c5fc.png)
 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
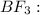
![{\text{Number of electron pairs}} =\frac{1}{2}[3+3-0+0]=3](/tpl/images/0137/9746/27036.png)
 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.