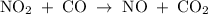The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no2 + no2 no + no3 step 2: no3 + co no2 + co2 the experimental rate law is rate = k[no2]2. (a) write the equation for the overall reaction. do not include phase abbreviations. (b) identify the intermediate(s). no3 no2 co no co2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

You know the right answer?
The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no...
Questions


Mathematics, 12.11.2020 20:20

Chemistry, 12.11.2020 20:20


History, 12.11.2020 20:20






Mathematics, 12.11.2020 20:20

Mathematics, 12.11.2020 20:20

Biology, 12.11.2020 20:20


Mathematics, 12.11.2020 20:20

Mathematics, 12.11.2020 20:20




Social Studies, 12.11.2020 20:20

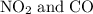 to produce NO and
to produce NO and 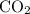 occurs in the given two steps.
occurs in the given two steps.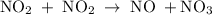

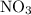 because it act as an intermediate and is utilized in the next step.
because it act as an intermediate and is utilized in the next step.