Chemistry, 27.07.2019 06:30 khikhi1705
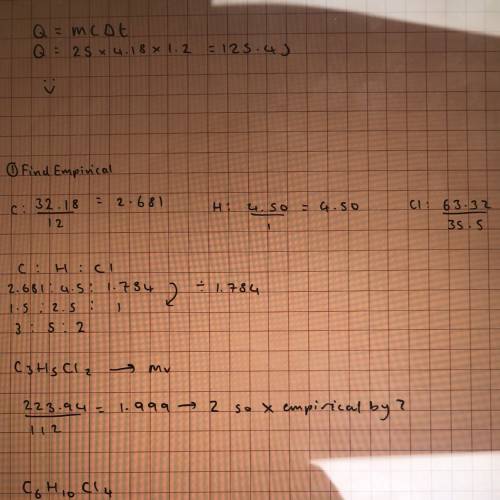
Molar mass of 223.94 g/mol consists of 32.18% c, 4.50% h, and 63.32% cl find the molecular formula for the compound

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Molar mass of 223.94 g/mol consists of 32.18% c, 4.50% h, and 63.32% cl find the molecular formula f...
Questions


Mathematics, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30



History, 13.07.2019 15:30




Mathematics, 13.07.2019 15:30

History, 13.07.2019 15:30



Biology, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30


Physics, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30

Mathematics, 13.07.2019 15:30