

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
Calculate the ph of 100 ml of a buffer solution containing 0.05 m benzoic acid (c6h5co2h; ka = 6.4...
Questions

Mathematics, 31.03.2020 20:51

Chemistry, 31.03.2020 20:51

Mathematics, 31.03.2020 20:51

Mathematics, 31.03.2020 20:51


World Languages, 31.03.2020 20:51



Mathematics, 31.03.2020 20:51


Mathematics, 31.03.2020 20:51





Mathematics, 31.03.2020 20:51

Mathematics, 31.03.2020 20:51


Mathematics, 31.03.2020 20:51

Mathematics, 31.03.2020 20:51

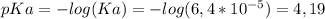 )
)![pH=pKa + log ( \frac{[A^{-}] }{[HA]} ) \\ \\ pH=4,19 + log ( \frac{0,05M}{0,05M}) \\ \\ pH=4,19](/tpl/images/0137/1803/3e5ee.png)


