
Chemistry, 27.07.2019 01:00 mamieengler
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1



Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of...
Questions

Computers and Technology, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10

Physics, 03.12.2020 07:10



Health, 03.12.2020 07:10


Spanish, 03.12.2020 07:10

Health, 03.12.2020 07:10

Computers and Technology, 03.12.2020 07:10

History, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10

English, 03.12.2020 07:10

Computers and Technology, 03.12.2020 07:10

History, 03.12.2020 07:10



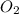 = 16 mole
= 16 mole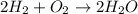
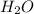
 moles of
moles of 

