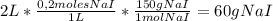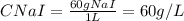Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Some amount of water is evaporated from a 2.0 l, 0.2 m nai solution, to from a 1.0 l solution. the m...
Questions

Computers and Technology, 14.02.2021 04:10

Computers and Technology, 14.02.2021 04:10

Mathematics, 14.02.2021 04:10


Mathematics, 14.02.2021 04:10

Mathematics, 14.02.2021 04:10


English, 14.02.2021 04:10

Mathematics, 14.02.2021 04:20


Mathematics, 14.02.2021 04:20

Health, 14.02.2021 04:20




Mathematics, 14.02.2021 04:20

Mathematics, 14.02.2021 04:20

Mathematics, 14.02.2021 04:20


Chemistry, 14.02.2021 04:20