

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Calculate the hydronium ion concentration in an aqueous solution with a ph of 9.85 at 25°c. calculat...
Questions

Computers and Technology, 02.12.2021 03:30


Mathematics, 02.12.2021 03:30









Mathematics, 02.12.2021 03:30


Physics, 02.12.2021 03:30


Computers and Technology, 02.12.2021 03:30

English, 02.12.2021 03:30

Mathematics, 02.12.2021 03:30

English, 02.12.2021 03:30

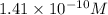
![pH=-\log [H_3O^+]](/tpl/images/0135/8173/841e8.png)
![9.85=-\log [H_3O^+]](/tpl/images/0135/8173/57f1c.png)
![[H_3O^+]=1.41\times 10^{-10}M](/tpl/images/0135/8173/012dc.png)


