Chemistry, 26.07.2019 17:30 haileysolis5
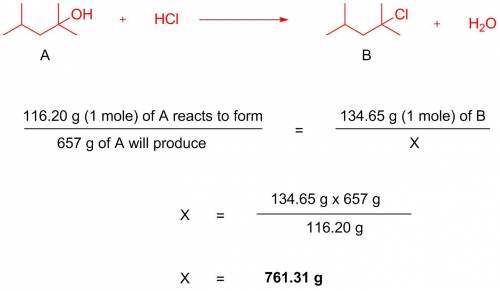
657 g of 2-chloro-2,4-dimethylpentane (g/mol = 134.65) is generated from a reaction between 2,4-dimethyl-2-pentanol (g/mol = 116.20) and excess hcl. assuming 100% yield, how many grams of 2,4-dimethyl-2-pentanol were used?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
657 g of 2-chloro-2,4-dimethylpentane (g/mol = 134.65) is generated from a reaction between 2,4-dime...
Questions




English, 22.09.2019 21:30



Physics, 22.09.2019 21:30

History, 22.09.2019 21:30

History, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30



Chemistry, 22.09.2019 21:30

History, 22.09.2019 21:30




Biology, 22.09.2019 21:30


English, 22.09.2019 21:30