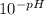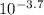Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

You know the right answer?
If the ph of a 1.00-in. rainfall over 1400 miles2 is 3.70, how many kilograms of sulfuric acid, h2so...
Questions

English, 22.12.2020 22:10

Mathematics, 22.12.2020 22:10







Mathematics, 22.12.2020 22:20

History, 22.12.2020 22:20

Computers and Technology, 22.12.2020 22:20

Mathematics, 22.12.2020 22:20

English, 22.12.2020 22:20



Mathematics, 22.12.2020 22:20

English, 22.12.2020 22:20