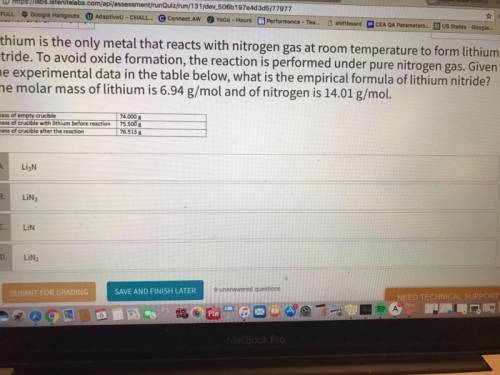
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride. to avoid oxide formation, the reaction is performed under pure nitrogen gas. given the experimental data in the table below, what is the empirical formula of lithium nitride? the molar mass of lithium is 6.94 g/mol and of nitrogen is 14.01 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride....
Questions






Mathematics, 26.06.2019 09:50


Mathematics, 26.06.2019 09:50


Mathematics, 26.06.2019 09:50






History, 26.06.2019 09:50


Mathematics, 26.06.2019 09:50