
Chemistry, 26.07.2019 15:30 CadenSkinner2003
A0.100 l solution of 0.300 m agno3 is combined with a 0.100 l solution of 1.00 m na3po4. calculate the concentration of ag and po43– at equilibrium after the precipitation of ag3po4 (ksp = 8.89 × 10–17).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
A0.100 l solution of 0.300 m agno3 is combined with a 0.100 l solution of 1.00 m na3po4. calculate t...
Questions


Mathematics, 05.06.2020 23:59









Mathematics, 06.06.2020 00:00

English, 06.06.2020 00:00







Health, 06.06.2020 00:00

Mathematics, 06.06.2020 00:00

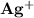 is 0.02998 M while the concentration of
is 0.02998 M while the concentration of 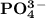 is 0.0999 M.
is 0.0999 M.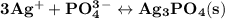
![\rm \bold{ Ksp = [ Ag^+]^3[ PO_4^3^- ]}](/tpl/images/0135/4274/43c55.png)
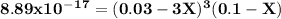
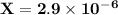
![\rm \bold{ [ Ag^+] = 0.02998 M}\\\\\\rm \bold{ [PO_4^3^-] = 0.0999 M }](/tpl/images/0135/4274/20a20.png)



