
Two moles of propene (c3h6) gas react with nine moles of oxygen (o2) gas to produce six moles of carbon dioxide gas (co2) and six moles of water gas (h2o). using this description of the reaction, write the corresponding chemical equation, showing the appropriate coefficients for each reactant and product. (include states-of-matter under the given conditions in your answer.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
Two moles of propene (c3h6) gas react with nine moles of oxygen (o2) gas to produce six moles of car...
Questions

Biology, 26.04.2021 21:30

Computers and Technology, 26.04.2021 21:30

Chemistry, 26.04.2021 21:30


History, 26.04.2021 21:30




World Languages, 26.04.2021 21:30

Social Studies, 26.04.2021 21:30

English, 26.04.2021 21:30

History, 26.04.2021 21:30

Mathematics, 26.04.2021 21:30

Mathematics, 26.04.2021 21:30



Computers and Technology, 26.04.2021 21:30


History, 26.04.2021 21:30

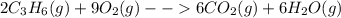
 to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.
to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.

