Chemistry, 26.07.2019 15:00 victorialeona81
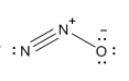
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance form is likely to contribute most to the correct structure of ? structure for nno showing three lone-pairs of electrons on the terminal nitrogen atom, a single bond between the two nitrogen atoms, a triple bond between nitrogen and oxygen, and one lone-pair of electrons on the terminal oxygen atom. structure for nno showing two lone-pairs of electrons on the terminal nitrogen atom, a double bond between the two nitrogen atoms, a double bond between nitrogen and oxygen, and two lone-pairs of electrons on the terminal oxygen atom. structure for nno showing one lone-pair of electrons on the terminal nitrogen atom, a triple bond between the two nitrogen atoms, a single bond between nitrogen and oxygen, and three lone-pairs of electrons on the terminal oxygen atom?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1
You know the right answer?
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance...
Questions

Mathematics, 19.01.2021 14:00





Health, 19.01.2021 14:00



Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Geography, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

English, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Computers and Technology, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

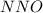 is:
is: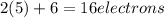
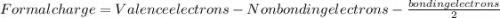
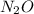 is:
is: