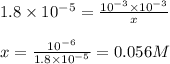Chemistry, 26.07.2019 15:00 lucerogon7403
Determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for the dissociation of nh3 (kb = 1.8 × 10-5) is determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for the dissociation of nh3 (kb = 1.8 × 10-5) is 1.8 × 10-2 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

You know the right answer?
Determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for...
Questions



Chemistry, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30






Advanced Placement (AP), 22.07.2019 17:30




Mathematics, 22.07.2019 17:30

English, 22.07.2019 17:30


Mathematics, 22.07.2019 17:30

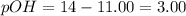
![pOH=-\log[OH^-]](/tpl/images/0135/3145/fe336.png)
![3.00=-\log [OH^-]](/tpl/images/0135/3145/bf5a2.png)
![[OH^-]=10^{-3}M](/tpl/images/0135/3145/d9858.png)
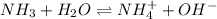
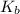 for above equation follows:
for above equation follows:![K_b=\frac{[NH_4^+][OH^-]}{[NH_3]}](/tpl/images/0135/3145/00f50.png)
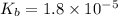
![[NH_4^+]=[OH^-]=10^{-3}M](/tpl/images/0135/3145/b4c85.png)