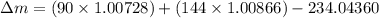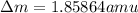Answer these questions based on 234.04360 as the atomic mass of thorium-234. the masses for the subatomic particles are given. round the mass defect to the 5th decimal place. mass of a proton: 1.00728 amu mass of a neutron: 1.00866 amu how many protons does thorium have? how many neutrons does thorium-234 have? calculate the mass defect for the isotope thorium-234 = amu

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
Answer these questions based on 234.04360 as the atomic mass of thorium-234. the masses for the suba...
Questions





Computers and Technology, 06.02.2021 18:20

Mathematics, 06.02.2021 18:20

Health, 06.02.2021 18:20


Business, 06.02.2021 18:20

Mathematics, 06.02.2021 18:20


Mathematics, 06.02.2021 18:20

Mathematics, 06.02.2021 18:20

Social Studies, 06.02.2021 18:20


Mathematics, 06.02.2021 18:20

Mathematics, 06.02.2021 18:20

Mathematics, 06.02.2021 18:20


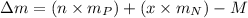
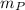 = mass of proton
= mass of proton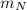 = mass of neutron
= mass of neutron