
Chemistry, 26.09.2019 01:00 fickllyd000
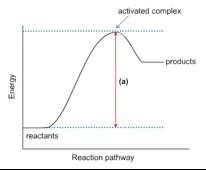
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as mercuric oxide) into liquid mercury and oxygen gas.
• write a balanced equation for the reaction.
• explain what feature is shown by the arrow labeled (a).
• using chemical symbols and dashed lines (this can be done with type), draw what the activated complex or transition state might look like.
• is this reaction exothermic or endothermic? explain.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1
You know the right answer?
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as m...
Questions





History, 26.04.2021 05:20


Chemistry, 26.04.2021 05:20


Health, 26.04.2021 05:20




History, 26.04.2021 05:20

Mathematics, 26.04.2021 05:20


Mathematics, 26.04.2021 05:20


Chemistry, 26.04.2021 05:20


Business, 26.04.2021 05:20



