
Chemistry, 26.12.2019 09:31 CrusaderLord
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) with 0.210 m koh(aq).
(a) before addition of any koh
(b) after addition of 25.0 ml of koh
(c) after addition of 35.0 ml of koh
(d) after addition of 50.0 ml of koh
(e) after addition of 60.0 ml of koh

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) wit...
Questions


English, 17.07.2019 00:50


Social Studies, 17.07.2019 00:50


Biology, 17.07.2019 00:50




Mathematics, 17.07.2019 00:50

Mathematics, 17.07.2019 00:50






Mathematics, 17.07.2019 01:00

History, 17.07.2019 01:00

Mathematics, 17.07.2019 01:00

![\text}{K}_{\text{a}} =\dfrac{ [H^{+}]^{2}}{\text{[HClO]}}](/tpl/images/0433/6132/82a3e.png)
![4 \times 10^{-8} =\dfrac{ [H^{+}]^{2}}{\text{[0.21]}}](/tpl/images/0433/6132/b84d3.png)
![[H^{+}]^{2} =4 \times 10^{-8} \times \text{0.21}](/tpl/images/0433/6132/f815a.png)
![[H^{+}]^{2} &= 8.4 \times 10^{-9 }](/tpl/images/0433/6132/4a2dc.png)
![[H^{+}] &= \sqrt{8.4 \times 10^{-9 }}](/tpl/images/0433/6132/0de98.png)
![[H^{+}] = 9.2 \times 10^{-5} \text{M}](/tpl/images/0433/6132/61a37.png)
![\text{pH} = - \text{log} [H^{+}]](/tpl/images/0433/6132/6fe5d.png)
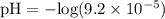
![\rm pH = Pka + log\dfrac{[Salt]}{[Acid]}](/tpl/images/0433/6132/d0693.png)
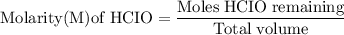
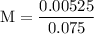
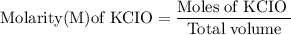
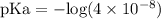
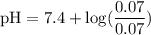
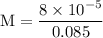
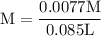
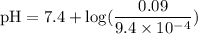
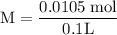
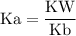
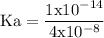
![\rm Kb = \dfrac {[CIO^{-}] [OH^{-}] }{ [KCIO]}](/tpl/images/0433/6132/0eaa1.png)
![\rm Kb = \dfrac {[OH^{-}] ^{2}}{ [KCIO]}](/tpl/images/0433/6132/2e104.png)
![\rm 2.5 x 10^{-7}= \dfrac {[OH^{-}] ^{2}}{ [0.105]}](/tpl/images/0433/6132/9ef1e.png)
![\rm pOH = - log [OH^{-}]](/tpl/images/0433/6132/91480.png)


