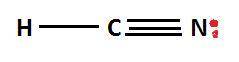Chemistry, 26.07.2019 04:00 melissa3333
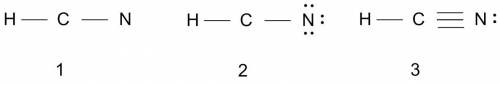
What is the lewis structure of the covalent compound that contains one nitrogen atom, one hydrogen atom, and one carbon atom? to add lone pairs, be sure to click the button lone-pair button before clicking on the molecule to add the lone pairs. use a line to connect each atom and indicate a bond. draw the molecule by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
What is the lewis structure of the covalent compound that contains one nitrogen atom, one hydrogen a...
Questions

Mathematics, 17.12.2020 01:20

Mathematics, 17.12.2020 01:20




English, 17.12.2020 01:20


English, 17.12.2020 01:20


History, 17.12.2020 01:20

Mathematics, 17.12.2020 01:20




Mathematics, 17.12.2020 01:20

Mathematics, 17.12.2020 01:20




Mathematics, 17.12.2020 01:20