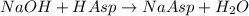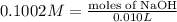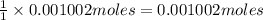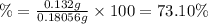Chemistry, 26.07.2019 03:30 codyshs160
Astudent found that the titration had taken 10.00 ml of 0.1002 m naoh to titration 0.132 g of aspirin, a monoprotic acid. calculate the percent purity of aspirin (c9h8o4, molar mass = 180.2 g/mol) sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
Astudent found that the titration had taken 10.00 ml of 0.1002 m naoh to titration 0.132 g of aspiri...
Questions

Computers and Technology, 27.08.2019 00:00





Social Studies, 27.08.2019 00:00

Biology, 27.08.2019 00:00




Computers and Technology, 27.08.2019 00:00

Mathematics, 27.08.2019 00:00


Spanish, 27.08.2019 00:00


History, 27.08.2019 00:00


Biology, 27.08.2019 00:00


Chemistry, 27.08.2019 00:00