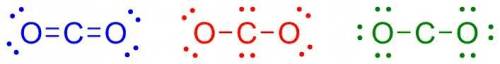Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond covalently, which of the following is the correct lewis dot (electron dot) structure for carbon dioxide? carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond covalently, which of the following is the correct lewis dot (electron dot) structure for carbon dioxide? a lewis dot structure with the following configuration: one oxygen atom with four valence electrons, a double bond to a carbon atom, followed by a double bond to a second oxygen atom which has four valence electrons. a lewis dot structure with the following configuration: one oxygen atom with four valence electrons, a single bond to a carbon atom with four valence electrons, followed by a single bond to a second oxygen atom which has four valence electrons. a lewis dot structure with the following configuration: one oxygen atom with six valence electrons, a single bond to a carbon atom, followed by a single bond to a second oxygen atom which has six valence electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2


Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond c...
Questions


Spanish, 18.02.2021 06:30

Mathematics, 18.02.2021 06:40

Mathematics, 18.02.2021 06:40


Mathematics, 18.02.2021 06:40




Mathematics, 18.02.2021 06:40





Mathematics, 18.02.2021 06:40

Mathematics, 18.02.2021 06:40

Mathematics, 18.02.2021 06:40


History, 18.02.2021 06:40