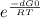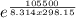Chemistry, 25.07.2019 14:30 michaelortega5808
When the oxide of generic metal m is heated at 25.0 °c, only a negligible amount of m is produced. o2m(s > m(s o2(g delta g= 288.9 kj/mol when this reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s. i got an answer of : o2m(s c(s > m(s co2(g this is correct. i need on this part of the question: what is the thermodynamic equilbrium constant for the coupled reaction? i attempted this twice and got k=1.22 and 0.89 and they are both wrong. ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
When the oxide of generic metal m is heated at 25.0 °c, only a negligible amount of m is produced. o...
Questions


Computers and Technology, 05.05.2020 16:00

Medicine, 05.05.2020 16:00




Social Studies, 05.05.2020 16:00

Mathematics, 05.05.2020 16:00

Mathematics, 05.05.2020 16:00

English, 05.05.2020 16:00

Medicine, 05.05.2020 16:01

English, 05.05.2020 16:01





Mathematics, 05.05.2020 16:01