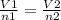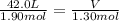Chemistry, 25.07.2019 14:00 brittany7436
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas (n1), and its volume is 42.0 l (v1). if 0.600 mole of gas leak out, and the pressure and temperature remain the same, what is the final volume of the gas inside the cylinder?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas...
Questions


Mathematics, 30.03.2021 22:40

Mathematics, 30.03.2021 22:40

Social Studies, 30.03.2021 22:40


Mathematics, 30.03.2021 22:40


History, 30.03.2021 22:40


English, 30.03.2021 22:40

Social Studies, 30.03.2021 22:40


Mathematics, 30.03.2021 22:40


Mathematics, 30.03.2021 22:40


Mathematics, 30.03.2021 22:40


Mathematics, 30.03.2021 22:40