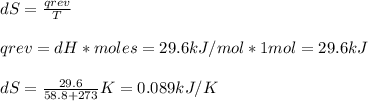Chemistry, 25.07.2019 11:00 kornut7316
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δhvap = 29.6 kj/mol. (a) when br2(l) boils at its normal boiling point, does its entropy increase or decrease? decrease (δs is negative) increase (δs is positive) (b) calculate the value of δs when 1.00 mol of br2(l) is vaporized at 58.8°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δh...
Questions


Biology, 13.11.2020 03:10

Chemistry, 13.11.2020 03:10



English, 13.11.2020 03:10


Mathematics, 13.11.2020 03:10

English, 13.11.2020 03:10






Chemistry, 13.11.2020 03:10

Mathematics, 13.11.2020 03:10