
Chemistry, 25.07.2019 07:30 ramentome7542
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of mgo is formed. what is the percent yield of this reaction? mg3n2 + 3 h2o --> 2 nh3 + 3 mgo

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of m...
Questions




Biology, 06.01.2021 18:00

Mathematics, 06.01.2021 18:00


Computers and Technology, 06.01.2021 18:00

Social Studies, 06.01.2021 18:00

Social Studies, 06.01.2021 18:00

History, 06.01.2021 18:00

Mathematics, 06.01.2021 18:00



Arts, 06.01.2021 18:00


Mathematics, 06.01.2021 18:00


Mathematics, 06.01.2021 18:00

English, 06.01.2021 18:00

History, 06.01.2021 18:00

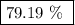 .
.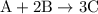
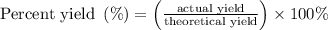 ......(1)
......(1)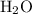 and is as follows:
and is as follows:
 ……. (2)
……. (2)

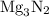 is
is  .
.
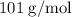 .
.
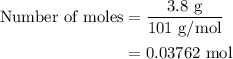
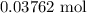 .
.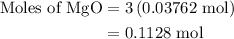

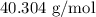 .
.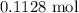 .
.
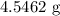 .
.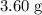 .
.
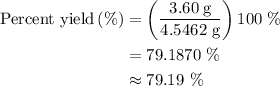
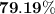 .
.


