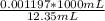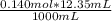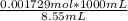Chemistry, 25.07.2019 07:00 hezekiahmharris
The initial volume of hcl was 1.25 ml and lioh was 2.65 ml. the final volume of hcl was 13.60 ml and lioh was 11.20 ml. if the lioh was .140 m what was the molarity of hcl ? if the same volumes were used from question 4, but the hcl was .140 m , what would the molarity of lioh be ?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
The initial volume of hcl was 1.25 ml and lioh was 2.65 ml. the final volume of hcl was 13.60 ml and...
Questions


Mathematics, 24.11.2020 01:10

Mathematics, 24.11.2020 01:10

English, 24.11.2020 01:10

English, 24.11.2020 01:10



Biology, 24.11.2020 01:10


Business, 24.11.2020 01:10

SAT, 24.11.2020 01:10

History, 24.11.2020 01:10

History, 24.11.2020 01:10

Advanced Placement (AP), 24.11.2020 01:10

Mathematics, 24.11.2020 01:10


Mathematics, 24.11.2020 01:10



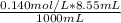 = 0.001197 mol
= 0.001197 mol