Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
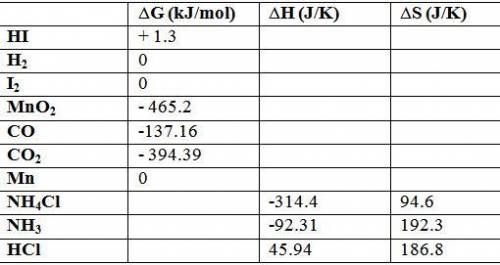
Calculate δg o for each reaction using δg of values: (a) h2(g) + i2(s) → 2hi(g) kj (b) mno2(s) + 2co...
Questions

Mathematics, 14.01.2020 23:31


Mathematics, 14.01.2020 23:31


Social Studies, 14.01.2020 23:31













Social Studies, 14.01.2020 23:31