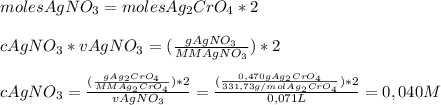We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble solid ag2cro4. when it has been dried and weighed, the mass of ag2cro4 is found to be 0.470 grams. what is the molarity of the agno3 solution? answer in units of m.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
You know the right answer?
We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble...
Questions

Geography, 05.08.2021 17:40


Mathematics, 05.08.2021 17:40

English, 05.08.2021 17:40






Mathematics, 05.08.2021 17:40

Mathematics, 05.08.2021 17:40




Mathematics, 05.08.2021 17:40


Mathematics, 05.08.2021 17:40