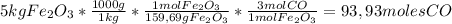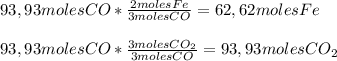Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
In a blast furnace, iron(iii) oxide is used to produce iron by the following (unbalanced) reaction....
Questions





Geography, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20






Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20