Chemistry, 25.07.2019 02:30 brittanyfox411
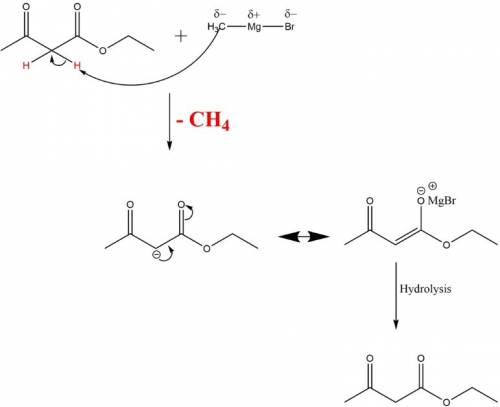
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evolved from the reaction mixture, and after adding aqueous acid, ethyl acetoacetate is recovered in high yield. identify the gas formed and explain why the starting material was recovered in this reaction. be sure to answer all parts. the gas formed is: o2 h2 ch4 co2 this occurs because: addition of a methyl group through nucleophilic addition destabilizes the ester group. because of the acidic proton, ch3mgbr reacts as a base and proton transfer occurs rather than nucleophilic addition. aqueous acid adds an oxygen to the carbanion formed releasing h2 gas. addition of aqueous acid disrupts the anhydride bond which reforms after decarboxylation?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evol...
Questions

Computers and Technology, 30.07.2019 23:10

Computers and Technology, 30.07.2019 23:10






Business, 30.07.2019 23:10

Business, 30.07.2019 23:10





Social Studies, 30.07.2019 23:10

Mathematics, 30.07.2019 23:10


Mathematics, 30.07.2019 23:10

English, 30.07.2019 23:10

Mathematics, 30.07.2019 23:10