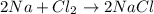Chemistry, 24.07.2019 16:30 tucecoskun
In the chemical reaction below, sodium (na) and chlorine (cl) combine to form sodium chloride (nacl). 2na + cl2 → 2nacl if the combined mass of the starting reactants is 50 grams, then the mass of sodium chloride formed once the reaction has reached completion a. will be exactly 50 grams. b. will be less than 50 grams. c. will be exactly 100 grams. d. will be between 50 and 100 grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
In the chemical reaction below, sodium (na) and chlorine (cl) combine to form sodium chloride (nacl)...
Questions

Geography, 05.10.2021 14:00


Mathematics, 05.10.2021 14:00


Mathematics, 05.10.2021 14:00




Mathematics, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00





Mathematics, 05.10.2021 14:00


Biology, 05.10.2021 14:00



Mathematics, 05.10.2021 14:00