
Chemistry, 24.07.2019 12:30 jayowens20
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the reaction rate, tripling the concentration of y triples the rate, and doubling the concentration of z has no effect. what is the rate law for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1
You know the right answer?
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the...
Questions





Mathematics, 27.07.2019 05:10

Mathematics, 27.07.2019 05:10







History, 27.07.2019 05:10





Mathematics, 27.07.2019 05:10

Mathematics, 27.07.2019 05:10

![\boxed{rate=k\left[ {{{\text{X}}_2}}\right]\left[ {\text{Y}} \right]}](/tpl/images/0127/2448/0a486.png) .
.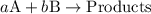
![{\text{rate}}=k{\left[{\text{A}}\right]^a}{\left[{\text{B}}\right]^b}](/tpl/images/0127/2448/dedd1.png) ...... (1)
...... (1)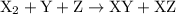
![{\text{rate}}=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}} \right]^c}](/tpl/images/0127/2448/0b279.png) …… (2)
…… (2)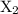 , Y, and Z respectively.
, Y, and Z respectively.![\begin{aligned}{\text{rate}}&=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}}\right]^c}\\&=k{\left[{{{\text{X}}_2}}\right]^1}{\left[ {\text{Y}}\right]^1}{\left[ {\text{Z}}\right]^0}\\&=k\left[{{{\text{X}}_2}}\right]\left[ {\text{Y}}\right]\\\end{aligned}](/tpl/images/0127/2448/fee78.png)



