
Chemistry, 24.07.2019 11:30 AshlynPlayz45
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2(g), would combine using to produce ammonia. the conditions included medium temperature (~500oc), very high pressure (~351kpa), and an iron catalyst. the reaction is represented by the equation: n2(g) + 3h2(g) → 2nh3(g) how many grams of nitrogen are needed to produce 100 grams of ammonia gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2...
Questions

Mathematics, 24.07.2019 12:00

Business, 24.07.2019 12:00




Mathematics, 24.07.2019 12:00

Mathematics, 24.07.2019 12:00


Biology, 24.07.2019 12:00


Mathematics, 24.07.2019 12:00



Chemistry, 24.07.2019 12:00



Mathematics, 24.07.2019 12:00


History, 24.07.2019 12:00

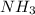 = 100 g
= 100 g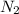 = 28 g/mole
= 28 g/mole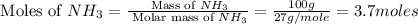
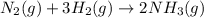
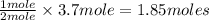 of
of 

