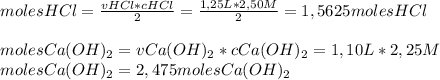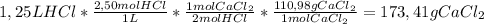Chemistry, 24.07.2019 08:00 freshysans4
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl) solution reacts with 1.10 liters of a 2.25 molar calcium hydroxide ca(oh)2 solution? (2 points) 2hcl (aq) + ca(oh)2 (aq) yields cacl2 (aq) + h2o (l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl)...
Questions

Biology, 22.01.2021 21:10


English, 22.01.2021 21:10



Mathematics, 22.01.2021 21:10


Mathematics, 22.01.2021 21:10

Mathematics, 22.01.2021 21:10



Medicine, 22.01.2021 21:10


Social Studies, 22.01.2021 21:10

English, 22.01.2021 21:10


Computers and Technology, 22.01.2021 21:10

History, 22.01.2021 21:10