Chemistry, 24.07.2019 06:30 Kangarue27
Suppose an oxygen-16 nuclide transforms into a nitrogen-13 nuclide by absorbing a proton and emitting an alpha particle. complete the nuclear chemical equation below so that it describes this nuclear reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Suppose an oxygen-16 nuclide transforms into a nitrogen-13 nuclide by absorbing a proton and emittin...
Questions




Biology, 26.03.2020 16:57

Physics, 26.03.2020 16:57







Chemistry, 26.03.2020 16:57

Mathematics, 26.03.2020 16:57



Mathematics, 26.03.2020 16:57

Mathematics, 26.03.2020 16:57



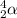 or
or 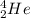 .
.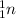 .
.