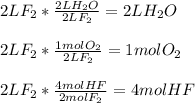When fluorine gas combines with water vapor, the following reactions occurs. 2f2(g) + 2h2o(g) → o2(g) + 4hf(g). if the reactant starts with 2 l of fluorine gas, how many liters of water vapor react with the fluorine, and how many liters of oxygen and hydrogen fluoride are produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
When fluorine gas combines with water vapor, the following reactions occurs. 2f2(g) + 2h2o(g) → o2(...
Questions

Mathematics, 09.02.2021 06:20

Mathematics, 09.02.2021 06:20




Chemistry, 09.02.2021 06:20


History, 09.02.2021 06:20






Mathematics, 09.02.2021 06:20



Geography, 09.02.2021 06:20



History, 09.02.2021 06:20