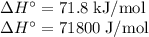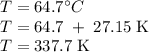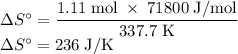Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
The normal boiling point of methanol is 64.7 °c and the molar enthalpy of vaporization if 71.8 kj/mo...
Questions



Health, 22.07.2019 09:10



History, 22.07.2019 09:10




Biology, 22.07.2019 09:10


Mathematics, 22.07.2019 09:10

Social Studies, 22.07.2019 09:10


Mathematics, 22.07.2019 09:10




History, 22.07.2019 09:20

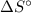 ) has been given as:
) has been given as: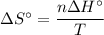
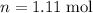
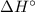 ),
),