Chemistry, 06.10.2019 01:50 Irishstoner5608
In 1864, the solvay process was developed to make soda ash. one step in the process is represented by the balanced equation below.
nacl + nh3 + co2 + h2o → nahco3 + nh4cl
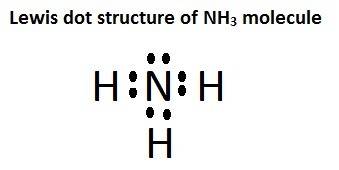
write the chemical formula for one compound in the equation that contains both ionic bonds and covalent bonds. explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule. in the space in your answer booklet, draw a lewis electron-dot diagram for the reactant containing nitrogen in the equation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
In 1864, the solvay process was developed to make soda ash. one step in the process is represented b...
Questions

English, 21.09.2019 01:30




Social Studies, 21.09.2019 01:30

Mathematics, 21.09.2019 01:30


English, 21.09.2019 01:30

Advanced Placement (AP), 21.09.2019 01:30

Biology, 21.09.2019 01:30

Mathematics, 21.09.2019 01:30

History, 21.09.2019 01:30

Social Studies, 21.09.2019 01:30

Mathematics, 21.09.2019 01:30

Chemistry, 21.09.2019 01:30

Mathematics, 21.09.2019 01:30



Mathematics, 21.09.2019 01:30

History, 21.09.2019 01:30

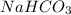
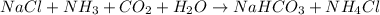
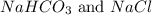 are ionic compounds and
are ionic compounds and 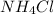 is a covalent compound.
is a covalent compound.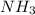 and the lewis dot structure of it is shown in the image attached.
and the lewis dot structure of it is shown in the image attached.