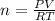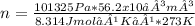Chemistry, 23.07.2019 17:00 elliedeegan3910
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mass in the sample. for the above problem how will you rearrange the ideal gas law to solve for moles of argon?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mas...
Questions


English, 19.04.2021 19:20

Mathematics, 19.04.2021 19:20

History, 19.04.2021 19:20




World Languages, 19.04.2021 19:20

Mathematics, 19.04.2021 19:20


Mathematics, 19.04.2021 19:20

Spanish, 19.04.2021 19:20

Chemistry, 19.04.2021 19:20


Mathematics, 19.04.2021 19:20

Social Studies, 19.04.2021 19:20

Mathematics, 19.04.2021 19:20