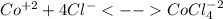In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(aq) is pink and cocl42-(aq) is blue. at low temperature the pink color predominates. at high temperature the blue color is strong. if we represent the equilibrium as: +(aq) + 4cl-(aq) cocl42-(aq) we can conclude that: 1. this reaction is: a. exothermic b. endothermic c. neutral d. more information is needed to answer this question.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

You know the right answer?
In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(...
Questions

Social Studies, 23.08.2019 10:30



English, 23.08.2019 10:30


Mathematics, 23.08.2019 10:30

Social Studies, 23.08.2019 10:30





History, 23.08.2019 10:30


Mathematics, 23.08.2019 10:30


Geography, 23.08.2019 10:30

Chemistry, 23.08.2019 10:30

Mathematics, 23.08.2019 10:30

Biology, 23.08.2019 10:30