Chemistry, 23.07.2019 16:30 fashionblogger28
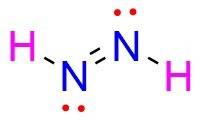
The lewis structure of n2h2 shows a. a nitrogen-nitrogen triple bond b. a nitrogen-nitrogen single bond c. each nitrogen has one nonbinding electron pair d. each nitrogen has two nonbinding electron pairs e. each hydrogen has one nonbonding electron pair

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
The lewis structure of n2h2 shows a. a nitrogen-nitrogen triple bond b. a nitrogen-nitrogen single...
Questions



Mathematics, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40




Mathematics, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40

Social Studies, 09.03.2021 20:40

History, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40

English, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40

Social Studies, 09.03.2021 20:40



Mathematics, 09.03.2021 20:40