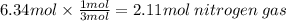Chemistry, 23.07.2019 07:30 valvaldeziv6373
The following balanced equation shows the formation of ammonia. n2 + 3h2 mc021-1.jpg 2nh3 how many moles of nitrogen are needed to completely convert 6.34 mol of hydrogen? 1.02 mol 2.11 mol 12.68 mol 19.02 mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
The following balanced equation shows the formation of ammonia. n2 + 3h2 mc021-1.jpg 2nh3 how many m...
Questions

Mathematics, 06.10.2019 22:30

Social Studies, 06.10.2019 22:30

History, 06.10.2019 22:30

Mathematics, 06.10.2019 22:30

Mathematics, 06.10.2019 22:30


Biology, 06.10.2019 22:30

Mathematics, 06.10.2019 22:30


Social Studies, 06.10.2019 22:30

Social Studies, 06.10.2019 22:30



Mathematics, 06.10.2019 22:30

Mathematics, 06.10.2019 22:30




History, 06.10.2019 22:30