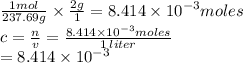Chemistry, 23.07.2019 06:00 helpmeplzandty
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what will be the molar concentration of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what...
Questions

English, 09.02.2021 17:30

Mathematics, 09.02.2021 17:30

Mathematics, 09.02.2021 17:30

History, 09.02.2021 17:30

Mathematics, 09.02.2021 17:30


Mathematics, 09.02.2021 17:30


Geography, 09.02.2021 17:30





History, 09.02.2021 17:30

History, 09.02.2021 17:30

History, 09.02.2021 17:30

Geography, 09.02.2021 17:30

Mathematics, 09.02.2021 17:30

Mathematics, 09.02.2021 17:30