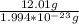Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

You know the right answer?
Consider a 12.01 g sample of carbon. the average mass of a carbon atom is 1.994 3 10223 g. how many...
Questions



Mathematics, 06.06.2021 23:50

History, 06.06.2021 23:50





Mathematics, 06.06.2021 23:50


Mathematics, 06.06.2021 23:50


Mathematics, 06.06.2021 23:50


Biology, 06.06.2021 23:50

Mathematics, 06.06.2021 23:50


Mathematics, 06.06.2021 23:50

Chemistry, 06.06.2021 23:50